Time, Temperature and Turbulence
Time
For example, in oil heat systems, the amount of time oil vapor has
to combust (or reside in the flame front or burning zone) has been improved dramatically
with the advent of flame retention burners. The
primary difference between this type burner and the older conventional style burner is
that the flame retention burners violently spin the air/fuel mixture resulting in better
mixing. This reduces the amount of excess
combustion air necessary to insure each fuel droplet is completely surrounded by oxygen
and burns completely. As the amount of
combustion air is reduced, efficiency increases.

O2 readings within the manufacturer’s specifications document that the burner’s flame retention properties are operating as designed and engineered.
With gas fired systems, air and fuel mixing occurs inside the burner. Again, increasing the amount of time the fuel and air have the opportunity to thoroughly mix will help insure complete combustion.

There is
a common misconception that the shutter on an atmospheric burner controls combustion air
intake. While some degree of combustion air
does enter through the shutters, draft remains constant. As the shutters are closed, more combustion air is drawn in through the
‘secondary’ air intake, thus, little combustion air control is provided.
Adjustable air intake shutters on
atmospheric burners primarily control the velocity at which the fuel/air mixture moves
down the burner throat or ‘mixing area’.
This can be demonstrated by taking a
combustion test while shutters are adjusted. Unless
the shutter is completely open or closed, little difference in the O2 / CO2 readings will be observed.
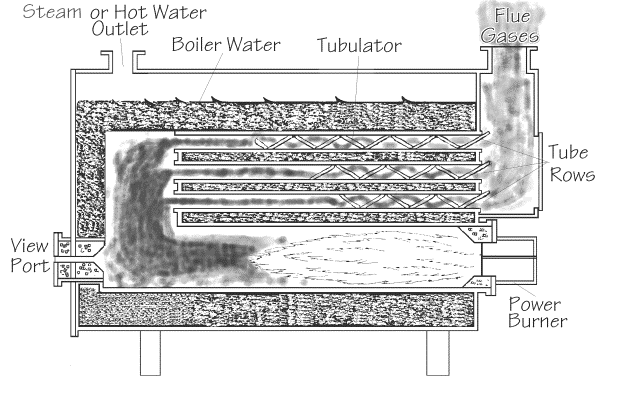
Increasing the amount of
time flue gases are in the heat exchanger also increases the amount of time for heat
transfer to occur.
O2 and stack temperature readings within the manufacturer’s specifications document that the flue gases are moving through the heat exchanger at a velocity which allows time for maximum heat transfer while still permitting the introduction of additional combustion air.
Temperature
As the temperature difference (DT or Delta T) between the source of heat and the material being heated increases, so does the rate of heat transfer. This heat transfer rate is measurable in forced air systems and boilers. By reducing the amount of combustion air introduced into the combustion process to the absolute minimum necessary, we increase the DT between the flame/flue gases and the distribution air or boiler water.
Radiant heat energy is much more effective in transferring heat than convective/conductive heat transfer. It is primarily put off by the flame itself and is directly related to flame temperature. As excess air is reduced, the higher flame temperature generates more radiant heat energy.
The burn rate in combustion process is very sensitive to temperature. If flame temperature is increased by 10%, the rate of combustion more than doubles. Unfortunately, the same increase in flame temperature also increases production of NOx gases by more than 10 times when sufficient O2 is available.

To estimate the actual flame
temperature, continue the horizontal line from the top of fuel to the point where it
intersects the line corresponding to the percent of excess air. From the point of intersection, draw a vertical
line up to the point where it intersects the combustion air temperature line (80°F);
Then draw a horizontal line to the vertical axis – temperature rise °F.
For example:
A natural gas burner operating with 25% excess air (4.5% O2) has an estimated flame temperature of 2930°F plus 80°F (combustion air temperature) equals 3010°F.
Note: Deduct approximately 11% for gas and 4% to 6% for oil due to water vapor in the flue gases.
Note that in this example
burning natural gas, a 25% excess air translates to a 4.5 O2 reading and a 20%
excess air translates to a 4.0% O2 reading on the analyzer. In other words, a ½ percent decrease in the O2 reading represents an almost 200° increase in flame temperature.
O2 readings within the
manufacturer’s specifications document that the flame temperature has been maximized
for the most efficient radiant heat production and transfer.
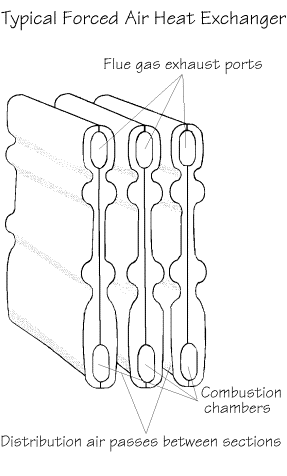
Turbulence
 Turbulation of the fuel, air and heat
source provides for more complete combustion by keeping these components in contact with
each other for a longer period of time.
Turbulation of the fuel, air and heat
source provides for more complete combustion by keeping these components in contact with
each other for a longer period of time.
Agitation of flue gases in a heat
exchanger serves to provide a continual circulation of hotter flue gasses in contact with
the heat exchanger surfaces. Typically, heat
exchanger surfaces have a wide variety of irregular surfaces incorporating bumps, ridges
etc. to provide this effect. Boilers and
domestic hot water heaters commonly have turbulators that provide for this mixing process. These surfaces also produce eddy currents that
recirculate flue gases and increase the amount of time those flue gases remain in the heat
exchanger.
These turbulators and irregular heat
exchanger surfaces are designed to be most effective at the appliance’s full firing
rate. Under firing a burner results in the
smaller volume of flue gases taking the path of least resistance through the boiler thus
reducing the scrubbing and mixing of the flue gases against the fire side of the heat
exchanger.
Again, O2 readings within
the manufacturer’s specifications verify that the burner is firing at full capacity,
maximizing efficient heat transfer.